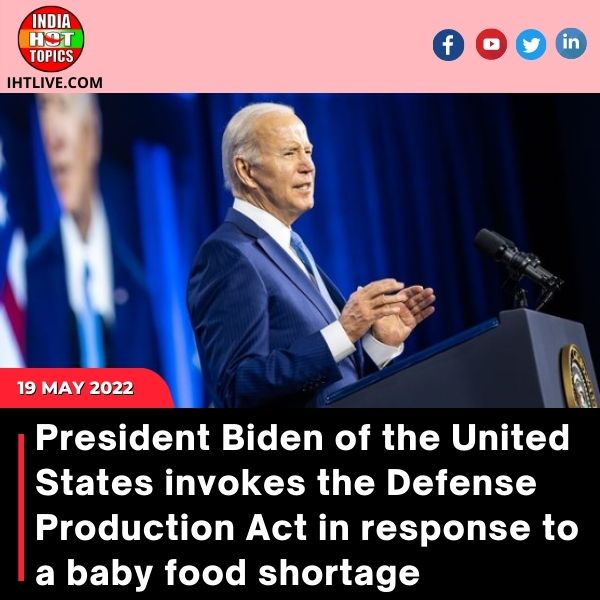
As he faces mounting political pressure over a domestic shortage caused by the safety-related closure of the country’s largest formula manufacturing plant, President Joe Biden invoked the Defense Production Act to speed up production of infant formula and authorised flights to import supply from overseas.
In order to avoid production bottlenecks, the Defense Production Act order requires formula manufacturers’ suppliers to fulfil orders before those of other customers. In what the White House is calling “Operation Fly Formula,” Biden is also authorising the Defense Department to use commercial aircraft to fly formula supplies that meet federal standards from overseas to the United States.
After a February recall by Abbott Nutrition exacerbated ongoing supply chain disruptions among formula makers, baby formula supplies across the country have been severely curtailed in recent weeks, leaving fewer options on store shelves and increasingly anxious parents struggling to find nutrition for their children.
In a video statement released by the White House on Wednesday, Biden said, “I know parents across the country are concerned about finding enough formula to feed their babies.” “I know how stressful that is as a parent and a grandparent.”
The news comes just two days after the FDA announced it was simplifying its review process to make it easier for foreign manufacturers to start shipping more formula into the United States.
Biden directed the Departments of Health and Human Services and Agriculture to work with the Pentagon over the next week to identify overseas supplies of formula that meet U.S. standards so that chartered Defense Department flights can quickly fly it to the United States.
“Baby formula imports will act as a bridge to this increased production,” Biden wrote.
Regulators announced on Monday that they had reached an agreement that would allow Abbott Nutrition to reopen its Sturgis, Michigan, plant, the country’s largest formula plant, which had been closed since February due to contamination concerns. Before resuming production, the company must overhaul its safety protocols and procedures.
Abbott estimates that it will take eight to ten weeks for new products to arrive in stores after receiving FDA approval. The company has not set a timetable for resuming production.
“I’ve directed my team to do everything possible to ensure that there is enough safe baby formula and that it reaches families in need as quickly as possible,” Biden said in a statement, calling it “one of my top priorities.”
The White House’s actions come as the Democratic-led House of Representatives approved two bills on Wednesday to address the baby formula shortage, as lawmakers seek to demonstrate progress on what has become a frightening development for many families.
A bill that received broad bipartisan support passed 414-9. It would allow the Agriculture Department’s secretary to issue a limited number of waivers in the event of a supply disruption. The goal is to allow participants in the WIC programme to use vouchers to buy formula from any manufacturer rather than being restricted to a single brand that may be unavailable. WIC accounts for roughly half of all infant formula sales in the United States.
“I want to assure the struggling mother that we hear her in Congress and that she does not have to deal with this alone.” We are working to find a solution for you “Rep. Jahana Hayes, D-Conn., is the bill’s sponsor.
The other bill, a $28 million emergency spending bill to boost resources at the Food and Drug Administration, passed 231-192, mostly along party lines, and it’s unclear whether the Senate will follow suit.
“This bill simply continues the Democrats’ strategy of throwing money at the same bureaucrats who created the crisis and have failed to make its resolution a top priority,” said one Democratic senator “Rep. Andy Harris, R-Md., agreed.
Rep. Rosa DeLauro, the Democratic chairwoman of the House Appropriations Committee, said the funds would be used to boost FDA staffing in order to improve inspections of domestic and international suppliers, prevent fraudulent products from reaching store shelves, and collect better market data.
House Speaker Nancy Pelosi, D-Calif., said, “It is critical that we ensure the federal government has the resources it needs to get baby formula back on the shelves.”
Four illnesses in babies who had consumed powdered formula from Abbott’s plant prompted the voluntary recall. Two of the four infants died after being hospitalised with a rare bacterial infection.
FDA investigators published a list of issues in March after a six-week inspection, including lax safety and sanitary standards and a history of bacterial contamination in several parts of the plant. Abbott must consult with an outside safety expert on a regular basis to restart and maintain production under the terms of Monday’s agreement.
Chicago-based Abbott has stated that their products have not been linked to bacterial infections in children. The bacteria found at its plant did not match the strains collected by federal investigators from two babies.
On a conference call with reporters on Monday, FDA officials rebuked the company’s reasoning for the first time publicly. Two of the four patients were unable to provide bacterial strains, limiting their chances of finding a match.
“We were limited in our ability to determine whether the product was linked to these four cases with a causal link right from the start because we only had sequences on two,” FDA food director Susan Mayne said.
According to former FDA officials, correcting the violations discovered at Abbott’s plant will take time. Companies must thoroughly clean their facilities and equipment, retrain their employees, and test and document that no contamination exists.
Companies will be required to provide documentation of their factory inspections as part of the FDA’s new import policy, according to regulators.

 Ranbir Kapoor1 month ago
Ranbir Kapoor1 month ago
 Mahakumbh1 month ago
Mahakumbh1 month ago
 American Dream4 weeks ago
American Dream4 weeks ago.jpg)
.jpg) Bollywood4 weeks ago
Bollywood4 weeks ago
 Sunny Leone1 month ago
Sunny Leone1 month ago
 Parineeti Chopra1 month ago
Parineeti Chopra1 month ago
 SSC Exam Calendar 20253 weeks ago
SSC Exam Calendar 20253 weeks ago
 Ajith Kumar4 weeks ago
Ajith Kumar4 weeks ago
.jpg)











.1.jpg)


.jpg)